Abstract
Background: BCMA, a member of the TNF receptor family, is expressed on MM and plasma cells. AMG 420, formerly BI 836909, binds BCMA on tumor cells and plasma cells and CD3 on T cells, resulting in T-cell mediated lysis of BCMA+ cells. Objectives of this study of AMG 420 in patients with R/R MM included assessing safety and tolerability and anti-tumor activity per IMWG 2006.
Methods: This is a FIH phase I dose escalation study (NCT02514239) of 6-week cycles of AMG 420 (1 cycle=4 weeks continuous IV infusion, 2 weeks off). Single-patient cohorts [0.2-1.6 µg/day (d)] were followed by cohorts of 3-6 patients (3.2-800 µg/d). Eligible patients had R/R MM and progression after ≥2 prior treatment lines (including proteasome inhibitor and immunomodulators); excluded were patients with plasma cell leukemia, extramedullary relapse, known central nervous system involvement, or prior allogeneic stem cell transplant. Treatment continued for up to 5 cycles or until disease progression (PD), start of new therapy, toxicity, withdrawal of consent, or investigator decision; 5 more cycles could be given per investigator for perceived benefit. MRD response was defined for this study as <1 tumor cell / 104 normal cells in the bone marrow per FACS using antibodies to cytIgλ, cytIgκ, CD19, CD56 or CD138, CD38, and CD45.
Results: As of May 22, 2018, 35 patients received AMG 420 (0.2-800 µg/d). Patients discontinued for PD (n=21), adverse events [AE, n=7, including 2 dose-limiting toxicities (DLTs)], or completed 10 cycles (n=2); 5 remain on study. Mean (SD) age was 63.8 (8.7) years, median 65 years, min-max 39-79 years, and 22 (63%) were male. Median MM disease duration was 5.4 (Q1: 3.3, Q3: 7.4) years, min-max 1.3-20 years, and median # of prior therapies was 4 (Q1: 2, Q3: 5), min-max 2-13. Patients (n=35) were treated for a mean (SD) of 2.3 (2.3) cycles and a median (min-max) of 1 (1-10) cycles; responders (n=8) were treated for a mean (SD) of 5.3 (3.3) cycles and a median (min-max) of 3.5 (2-10) cycles, including those with treatment ongoing.
Regarding safety, one patient in the 50 µg/d cohort died after the first cycle from acute respiratory distress due to concurrent flu and aspergillosis not considered related to treatment. Of those with serious AEs (n=17, 49%), 12 required hospitalization and another 3 had prolonged hospitalization. Serious AEs included infections (n=10, 29%, 3 device-related, 3 pneumonias, 2 catheter site, 1 aspergillus, 1 influenza, and 1 fever/infection), cytokine release syndrome (CRS, n=3), and 1 each of peripheral polyneuropathy (PPN), cardiac failure, edema, pyrexia, biliary obstruction, and renal failure. Treatment-related serious AEs included CRS (n=3, 2 grade 1 and 1 grade 3) and 1 each of PPN (grade 3), edema (grade 3), and pyrexia (grade 1). No anti-AMG 420 antibodies were detected up to 800 μg/d and no DLTs were observed up to 400 µg/d. In this study, 800 µg/d was determined to not be tolerable as 2/3 patients experienced DLTs: 1) Grade 3 CRS within 1d of initiating treatment with fever, hypertension, tachycardia, and retrograde amnesia; symptoms resolved after stopping drug, and 2) Grade 3 PPN that required hospitalization with subsequent complete recovery; after 15d of treatment, M protein decreased by 60%.
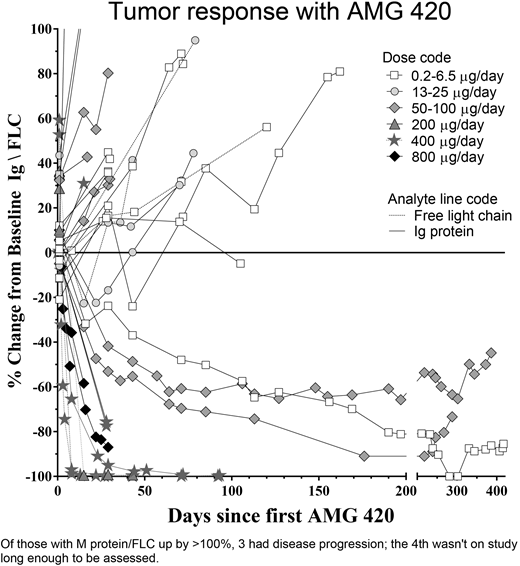
Six patients had complete responses (CRs), 1 each at 6.5, 100, and 200 µg/d, and 3 at 400 µg/d; responses are ongoing for these last 3 (≥4.6 months). There also were two partial remissions, a partial response (PR) at 50 µg/d and a very good partial response at 800 µg/d. Response duration was for up to 8 cycles (1 patient had PR cycles 3-10). All patients at 400 µg/d (3/3) had MRD negative CRs. In the dose confirmation cohort enrolled after May 22, 2 of 3 patients had PRs as of cycle 1. Thus, at the dose of 400 µg/d, the objective response rate is 5/6 (83%); all 5 are still responding on treatment. Pharmacokinetic analyses show that responders had higher free-drug exposure levels than non-responders [median (range) of 3,225 (36-108,000) vs 97 (27-1,380) pg/mL].
Conclusions: In this FIH study, AMG 420, a short half-life BiTE® targeting BCMA, showed encouraging evidence of activity in patients with R/R multiple myeloma. During dose escalation, all 3 patients dosed with 400 µg/d had MRD-negative CRs, with 2 more responders in the dose confirmation cohort to date; 3 patients at lower doses also attained CRs. No major toxicities were observed up to 400 µg/d, which is a recommended dose for further investigation; DLTs at 800 µg/d were CRS and PPN.
Topp:Regeneron Pharmaceuticals, Inc.: Honoraria, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Research Funding. Zugmaier:Amgen Inc.: Consultancy, Employment, Patents & Royalties: 20170327581, 9688760, 20170122947, 9486475, 20160208001, 9192665, 20150071928, 8840888, 20140227272, 20140228316, 20130323247, 20130287774, 20130287778, 20110262440, 20100112603, 7700299, 20070037228. Moreau:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Facon:Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Oncopeptides: Membership on an entity's Board of Directors or advisory committees. Munzert:Boehringer Ingelheim: Employment, Patents & Royalties: and other intellectual property .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal